SUZHOU,China,Nov. 4,2024 -- According to the latest data from the Maternal and Child Health Department of the Chinese National Health Commission,there are currently 602 medical institutions in China that have been approved to carry out human assisted reproductive technology,and the number of IVF institutions worldwide is also continuously increasing. These institutions mainly use computer-aided sperm analysis (CASA) methods to analyze indicators such as sperm quantity,vitality,and morphology in sperm testing,in accordance with the WHO Fifth Edition guidelines. From the demand side,the decline in sperm quality among Chinese men has become an undeniable fact. The Frost Sullivan report points out that in the past 40 years,the sperm count of Chinese men has decreased by as much as 75%,and the proportion of infertility caused by male factors is close to 40%.
According to data from China Intelligent Research Consulting,the market size of sperm quality analyzers in China will continue to expand in the coming years. In 2023,its scale has approached 300 million RMB,and the global market size of sperm analyzers is as high as 400 million US dollars. What is even more worth looking forward to is that if developed at a compound growth rate of 10%,the global market size will exceed 500 million US dollars by 2026. This is a market with enormous potential,like a gold mine waiting to be excavated,full of opportunities.

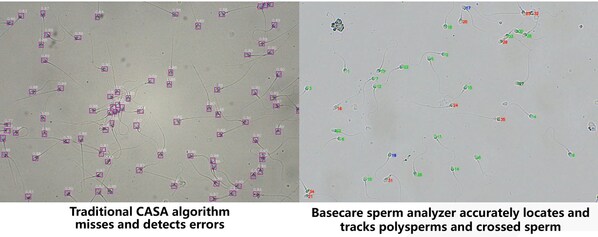
However,traditional CASA sperm detection techniques have significant limitations. On the one hand,its detection reliability and repeatability are poor,and it cannot detect the morphology of live sperm. On the other hand,CASA requires staining of sperm samples during the operation process and relies on manual operation,which not only increases the complexity of detection,but also may alter the morphology of sperm during the staining process,thereby affecting the accuracy of detection results. In response to these pain points,Basecare Medical,in collaboration with 18 leading IVF centers in China,has launched the largest AI sperm testing research in China. They have completed detailed comparisons and clinical validations of 500000+ sperm samples and developed automated live sperm testing technology,aiming to break through the limitations of traditional CASA technology and improve the accuracy and efficiency of sperm testing.
In addition,in the application of traditional CASA technology,patients have to travel to and from the hospital at least twice,and sometimes even wait for a week to receive the test report. In contrast,Basecare Medical's intelligent sperm quality analyzer uses AI technology for analysis,without the need for staining sperm samples,and can complete the analysis of the morphology,concentration,and vitality of unstained live sperm within 3 minutes at the fastest. Patients only need to undergo one test to identify qualified sperm that can be used for embryo culture,greatly optimizing their service experience. By reducing the number of tests and shortening waiting times,the Basecare sperm analyzer not only improves patient satisfaction,but also significantly enhances hospital efficiency and shortens the overall duration of IVF cycles.
The latest announcement shows that the intelligent sperm quality analyzer (BKA210) from Basecare Medical has recently been approved for market (NMPA number: 20242222101),which is the world's first device capable of detecting unstained live sperm. This device has been awarded the National Disruptive Technology Innovation Excellent Project Award by the country for its domestically pioneering and internationally leading innovative technology,which means that the product has been highly recognized by Chinese government and clinical experts. From the interim report of Basecare,it can be seen that it has received a large number of orders in the field of andrology laboratory,and its commercial strength after certification cannot be underestimated.

Basecare Medical Intelligent Sperm Analyzer (NMPA number: 20242222101)
Overall,the approved sperm analyzer from Basecare Medical,combined with the company's globally leading Geri ® Time-Lapse incubator and iARMS intelligent assisted reproductive management system are gradually building a comprehensive ecosystem of intelligent assisted reproductive centers. As Basecare Medical continues to strengthen its men's health product line,the company is directly improving clinical testing results from the product end,committed to creating truly innovative and competitive products in the market. This innovation not only improves the accuracy and efficiency of detection,but also has the potential to bring significant improvements in performance,which in turn translates into sales growth. In addition,Basecare Medical's sperm analyzer is applying for CE certification for the European market. It is expected that after obtaining CE certification by the end of the year,it will further expand its global market and continue to make positive contributions to the reproductive health industry in China and even globally.